
Empirical Formula Worksheet Pdf worksheet
Formula Practice worksheets We came up with two significant sorts of chemical formulas: molecular formula and empirical formula, by calculating the masses of all the constituent atoms that combine to create a molecule.

Percent Composition By Mass Worksheet
1) 26.4 % Carbon 3.3 % Hydrogen 70.3 % Oxygen Molar Mass: 91.0 g/mol Empirical Formula Molecular Fornmila: 2) 81.8 grams Carbon 18.2 grams Hydrogen Molar Mass: 132.0 g/mol This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Empirical And Molecular Formulas Worksheet
What is the empirical formula of the product? Chapter 7 - The Simplest, or Empirical, Formula Section A Determine the empirical formula for each compound whose percentage composition is shown below. 43% C and 57% O 40.3% K, 26.7% Cr, and 33.0% O 32.0% C, 42.6% O, 18.7% N, and the remainder H 31.9% K, 28.9% Cl, and the remainder O

SOLVED Empirical and Molecular Formula Worksheet Show ALL your work
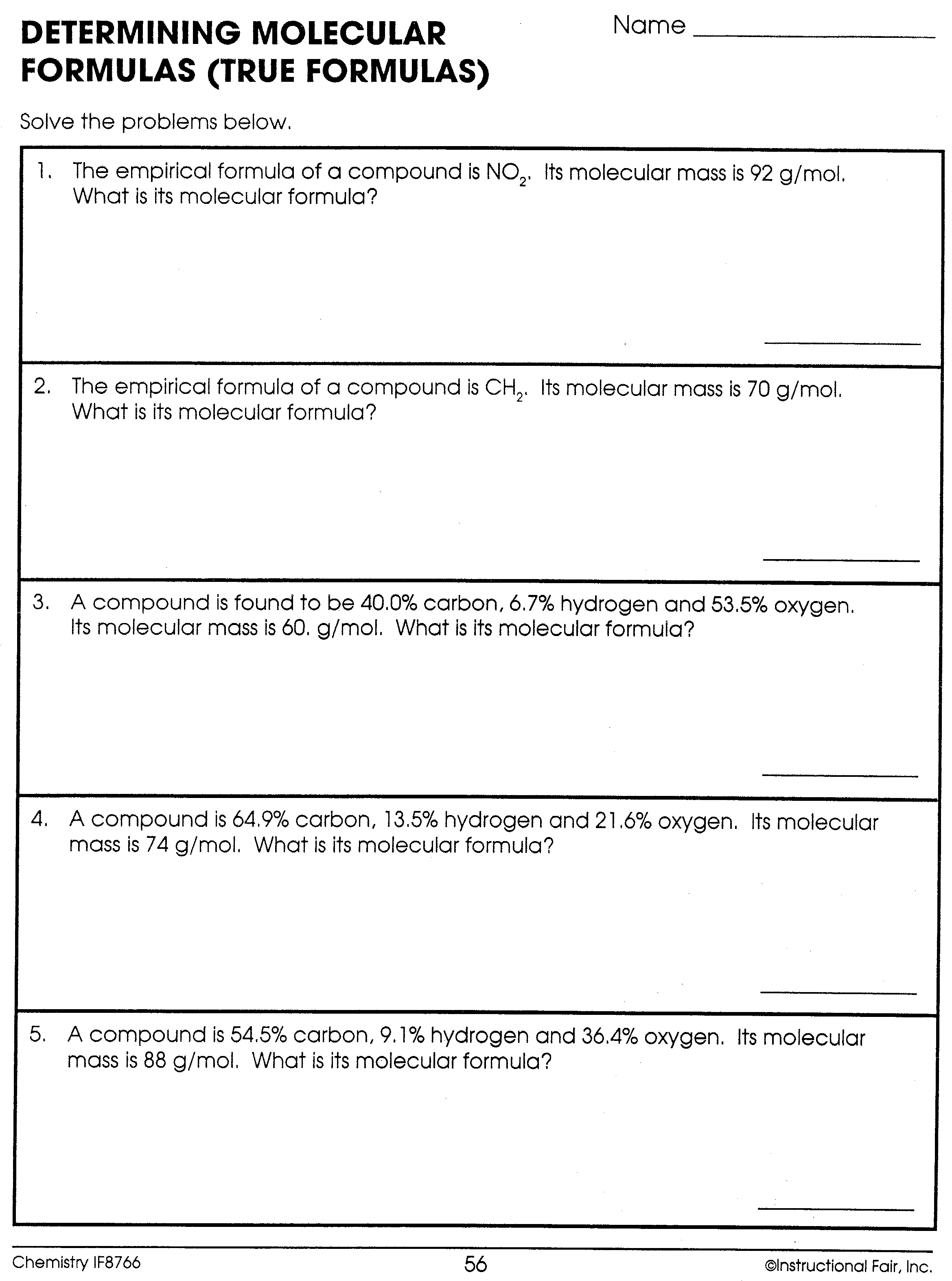
This 10-question practice test deals with finding the molecular formula of chemical compounds. A periodic table will be required to complete this test. Answers appear after the final question. Question 1 An unknown compound is found to contain 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen with a molecular mass of 60.0 g/mol.

formula writing worksheet answers
1) 26.4% Carbon 3.3 % Hydrogen 70.3 % Oxygen Molar Mass: 91.0 g/mol Empirical Formula: Molecular Formula: 2) 81.8 grams Carbon 18.2 grams Hydrogen Molar Mass: Here's the best way to solve it. Empirical/Molecular Formula Practice Worksheet Directions: - questions to Find the empirical AND molecular formulas given the percent compositions or.
Empirical And Molecular Formula Problems Pdf UPDATED
View Empirical Formula Practice 1 (1).docx from CHEM 3332 at University of Houston. Name: Period: Date: Score: Empirical/Molecular Formula Practice Worksheet Directions: Find the empirical AND

Empirical And Molecular Formulas Worksheet
Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Answer . Mg 3 Si 2 H 3 O 8 (empirical formula), Mg 6 Si 4 H 6 O 16 (molecular formula)

Empirical and Molecular Formula Practice by Teach Simple
Empirical and Molecular Formulas. Exercise 7.2.3.1 7.2.3. 1. For each formula below, give the empirical formula. Sometimes the formula given is the same as the empirical, sometimes it is different. a) C 3 H 6 O 3. b) N 2 O 4. c) Mg 3 N 2. d) C 7 H 14 O 2. e) P 2 O 5.

8 Best Images of Percent Composition Worksheet Answer Key Percent
Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Answer . Mg 3 Si 2 H 3 O 8 (empirical formula), Mg 6 Si 4 H 6 O 16 (molecular formula)

Empirical and Molecular Formula Practice Solutions Docsity
Empirical/Molecular Formula Practice Worksheet Directions: Find the empirical AND molecular formulas given the percent compositions or masses. SHOW YOUR WORK to receive full credit. 1) 26.4 % Carbon 3.3 % Hydrogen 70.3 % Oxygen Empirical Formula:_________________ 2) 81.8 grams Carbon 18.2 grams Hydrogen Empirical Formula:_________________

Determining Empirical Formulas Worksheet Printable Word Searches
C2H2Cl2. A compound is contains 46.7g nitrogen and 53.3g oxygen. If the molecular mass of the compound is 60.0 g/mol, what is the molecular formula? N2O2. Study with Quizlet and memorize flashcards containing terms like 40.05g S and 59.95g O. Find the empirical formula for these elements., Given the following: 42.07g Na, 18.89g P, and 39.04g O.

Empirical Formula Practice Worksheet
The molecular mass of the compound is 58.12 g/mol. Determine the molecular formula. 8 min max PAGE 349, QUESTION 145 145) Determine the molecular formula for ibuprofen, a common headache remedy. Analysis of ibuprofen yields a molar mass of 206 g/mol and a percent composition of 75.7% C, 8.80% H, and 15.5% O. Determine the molecular formula. 10.
Empirical Formula Worksheet 1 Answers
Empirical/Molecular Formula Practice Worksheet Directions: Find the empirical AND molecular formulas given the percent compositions or masses. SHOW YOUR WORK to receive full credit 1) 26,4% Carbon 3.3 % Hydrogen 70.3 % Oxygen Molar Mass: 91.0 g/mol Empirical Formula: Molecular Formula: 2) 81.8 grams Carbon 18.2 grams Hydrogen Molar Mass: 132,0.

Empirical/molecular Formula Practice Worksheet Answer Key Printable
Objectives: • be able to calculate empirical and molecular formulas Empirical Formula 1) What is the empirical formula of a compound that contains 0.783g of Carbon, 0.196g of Hydrogen and 0.521g of Oxygen? 2) What is empirical formula of a compound which consists of 89.14% Au and 10.80% of O?

Solved Empirical/Molecular Formula Practice Worksheet
Worksheet: Calculating Empirical & Molecular Formulas 1. The empirical formula for the compound having the formula H2C2O4 is [A] C2H2 [B] CO2H [C] COH [D] C2O4H2 [E] COH2 2. Calculate the empirical formula of a compound that is 85.6% C and 14.4% H (by mass). [A] CH2 [B] CH [C] C3H5 [D] C2H4 [E] C2H 3.

Empirical and Molecular Formula Worksheet Write the empirical formula
Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER. Write the empirical formula for the following compounds. 1) C 6H 6 2) C8H18 3) WO2 4) C2H6O2 5) X 39Y 13 6) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. What is the molecular formula of this compound?